Менингококковая инфекция (МИ) не теряет своей актуальности и продолжает доставлять беспокойство органам и структурам здравоохранения, в том числе на глобальном уровне, несмотря на более чем пятидесятилетние усилия по разработке и применению эффективных менингококковых вакцин [1, 2]. К настоящему времени в мире активно применяются конъюгированные полисахаридные вакцины для 4 (ACWY) из 6 наиболее опасных серогрупп Neisseria meningitidis (ABCWYX) [3, 4]. Кроме того, конъюгированная пятивалентная вакцина, включающая серогруппу X, находится во II фазе клинических испытаний [5]. Такие конъюгированные вакцины эффективны у младенцев, способствуют более продолжительному иммунному ответу и могут вызывать коллективную защиту, снижая передачу возбудителя среди людей. Разработка вакцин на основе полисахарида против Neisseria meningitidis серогруппы B оказалась неприемлемой из-за плохой иммуногенности полисахаридной капсулы серогруппы B и проблем с аутоантигеном [6]. В поисках потенциальных субкапсулярных антигенов для вакцины против серогруппы B важную роль сыграла геномика. С использованием доступного полного генома Neisseria meningitidis и подхода, называемого «обратной вакцинологией» [7], были идентифицированы кандидаты в состав белковой вакцины. Выбранные мишени, в том числе нейссерийный адгезин A (NadA – Neisseria adhesin A), нейссерийный гепарин-связывающий белок (NHBA – Neisserial Heparin Binding Antigen) и фактор Н-связывающий белок (fHbp – factor H binding protein), были объединены с везикулами наружной мембраны (OMV) новозеландского штамма NZ98/254, содержащего PorA P1.4 [3] для получения вакцинного препарата Bexsero [8]. Учитывая известное разнообразие последовательностей fHbp [9], была разработана другая белковая В-менингококковая вакцина – Trumenba, для охвата двух подсемейств fHpb, что позволяло потенциально воздействовать на все Neisseria meningitidis серогруппы B, а также на другие серогруппы [10]. Принимая во внимание разнообразие антигенов в менингококковой популяции, были выполнены крупные международные проекты по секвенированию штаммов менингококка, циркулирующих в разных географических районах, для оценки их охвата В-менингококковыми вакцинами [11, 12]. Проведенные исследования показали, что полногеномное секвенирование позволяет эффективно охарактеризовать любой набор генов, кодирующих антиген, в большой коллекции изолятов и дает возможность выявлять потенциальный охват циркулирующих штаммов различными вакцинами. Ценность полногеномного секвенирования заключается в определении изменений в распространенности вакцинных антигенов после введения вакцины и идентификации возникающих и ускользающих мутантных штаммов менингококка.
Ежегодно в мире возникает 1 млн случаев генерализованной формы МИ (ГФМИ) [13]. Общая летальность колеблется от 4 до 20%, в зависимости от серогруппы штамма и возраста человека [14]. Самая высокая заболеваемость выявлена у детей в возрасте до 1 года, а половина всех случаев приходится на детей в возрасте до 5 лет. Второй пик заболеваемости наблюдается у подростков и молодых взрослых, то есть среди лиц с наибольшей распространенностью назофарингеального носительства [13, 15]. Отличия МИ от других инфекционных заболеваний многочисленны, и едва ли возможно перечислить их все без исключения. Так, случаи заболеваний, вспышки, эпидемии, пандемии возникают внезапно; эпидемиология МИ динамична и непредсказуема; длительные интервалы между случаями заболеваний затрудняют прогнозирование течения эпидемического процесса МИ. До сих пор нет ответа на вопрос, почему заболевание возникает у одних лиц и не возникает у других, при этом известно, что возбудитель (Neisseria meningitidis) в виде бессимптомного назофарингеального носительства повсеместно распространен и с менингококком может встретиться каждый. Уровень носительства среди населения составляет 2–10%, однако случаи ГФМИ при спорадической заболеваемости единичны и не имеют доказанной взаимосвязи. Для инфицирования и заболевания необходим комплекс факторов: вирулентный возбудитель, внешние факторы риска, восприимчивый организм и в меньшей степени другие факторы. Заболевание «обрушивается» на человека неожиданно, при этом развитие его происходит на фоне полного здоровья человека, и начальные симптомы могут быть неспецифичны (ОРЗ, ОРВИ, кишечная инфекция и др.). Точный диагноз при этом поставить сложно или невозможно, упускается время для начала адекватного лечения, и в этой связи может возрастать вероятность летального исхода, возникновения осложнений и последствий. Особая опасность – развитие гипертоксической (молниеносной) формы болезни, которая может закончиться летальным исходом в первые несколько часов (6–24 ч) после начала заболевания. Исход заболевания непредсказуем. Так, в современных условиях наиболее частый исход – полное выздоровление. Однако может быть выздоровление с осложнениями разной степени тяжести на разных стадиях болезни, выздоровление с последствиями разной степени тяжести после болезни и летальный исход. Чрезвычайно важной и малоизученной проблемой является стоимость заболевания, особенно если учитывать совокупную стоимость лечения в стационаре и затраты на реабилитацию при возникновении инвалидизирующих последствий от заболевания. Специальными исследованиями показана высокая степень обремененности от заболевания с точки зрения инвалидности и расходов на медицинское страхование, расходов семьи и расходов государственных учреждений. Установлено, что при разработке и обосновании стратегий профилактики важно учитывать все параметры течения болезни, а именно острый период заболевания, осложнения и последствия с точки зрения экономических затрат. Финансовые ежегодные пожизненные траты переболевшего ГФМИ на последствия от заболевания наглядно представлены в некоторых специально проведенных уникальных исследованиях. Так, в работе французских исследователей [16] проведена оценка стоимости эпизодов молниеносной формы менингококцемии и менингококкового менингита с тяжелыми последствиями, и описаны затраты на последствия от заболевания по годам. В работе представлено 2 сценария.
Первый сценарий: пациент А., мальчик 6 лет. Диагностирована молниеносная форма менингококцемии, заболевание осложнилось, проведена ампутация обеих ног ниже колен. На протяжении всей последующей жизни рассчитаны общие финансовые траты на последствия заболевания от начала симптомов до смерти в 77 лет, составившие 1 453 492 евро. Наивысшие затраты пришлись на первый год после начала заболевания, составившие 160 000 евро. Категории самых значимых трат: протезы, специализированное оборудование, образование, больничный уход, реабилитационная помощь, амбулаторная помощь.
Второй сценарий: пациент В., девочка 3 лет. Поставлен диагноз менингита, осложнившегося тяжелыми неврологическими последствиями. Рассчитана общая стоимость затрат от начала симптомов до смерти в 55 лет, составившая 3 921 587 евро. Наивысшие затраты пришлись на первый год после начала заболевания и составили 160 000 евро. Категории самых значимых трат: образование, попечение по месту жительства, специализированное оборудование, больничный уход.
Аналогичное исследование проведено в Великобритании в 2013 г. Фондом исследования менингита (Meningitis Research Foundation – MRF). Было подсчитано, что общая стоимость одного тяжелого случая ГФМИ для правительства Великобритании в течение 70 лет составляет от 1 360 000 до 1 720 000 фунтов стерлингов [17]. MRF также оценила, что тяжелые случаи ГФМИ могут стоить от 160 000 до 200 000 фунтов стерлингов на одного больного в первый год после начала заболевания. Эти данные согласуются с результатами исследования испанских специалистов, проведенного по сходной методике [18]. Данные испанского исследования показали общую стоимость трат на одного больного 1 200 000 евро и 1 400 000 евро за 70 лет. По их оценкам, случаи ГФМИ связаны со стоимостью 197 000 и 155 000 евро на одного больного в первый год после начала заболевания.
В Российской Федерации экономический ущерб от МИ в 2019 г. составил в общей сложности 372 млн руб. [19] и не включал пожизненные траты на реабилитационные мероприятия в случае инвалидности от заболевания. Единственный путь предотвращения трагических событий и экономических потерь, связанных с МИ, – упреждающая вакцинопрофилактика. Вакцинопрофилактика МИ в РФ регламентируется Приказом Министерства здравоохранения РФ № 1122н от 06.12.2021 в части «Календарь прививок по эпидемическим показаниям» с формулировкой: «прививаются дети и взрослые в очагах менингококковой инфекции, вызванной менингококками серогруппы А или С, вакцинация проводится в эндемичных регионах, а также в случае эпидемии, вызванной менингококками серогрупп А или С, прививаются лица подлежащие призыву на военную службу»1. Данная формулировка нуждается в корректировке из-за изменившихся современных реалий. Так, согласно новому федеральному документу СанПиН 3.3686-21 «Санитарно-эпидемиологические требования по профилактике инфекционных болезней» (раздел XXXIX «Профилактика менингококковой инфекции»), вакцинации подлежат «группы риска по инфицированию и заболеванию: а именно, вакцинируются лица, подлежащие призыву на военную службу; лица, отъезжающие в эндемичные по менингококковой инфекции районы (например, паломники, военнослужащие, туристы, спортсмены, геологи, биологи); медицинские работники структурных подразделений, оказывающих специализированную медицинскую помощь по профилю «инфекционные болезни»; медицинские работники и сотрудники лабораторий, работающих с живой культурой менингококка; воспитанники и персонал учреждений стационарного социального обслуживания с круглосуточным пребыванием (дома ребенка, детские дома, интернаты); лица, проживающие в общежитиях; лица, принимающие участие в массовых международных спортивных и культурных мероприятиях; дети до 5 лет включительно (в связи с высокой заболеваемостью в данной возрастной группе); подростки в возрасте 13–17 лет (в связи с повышенным уровнем носительства возбудителя в данной возрастной группе); лица старше 60 лет; лица с первичными и вторичными иммунодефицитными состояниями, в том числе ВИЧ-инфицированные; лица, перенесшие кохлеарную имплантацию; лица с ликвореей». Кроме того, экстренная вакцинация показана в очагах МИ и среди контактных с больным лиц2. Целесообразной и необходимой является гармонизация двух федеральных документов и внесение перечня групп риска в национальный календарь профилактических прививок в части «Календарь профилактических прививок по эпидемическим показаниям» для охвата вакцинацией наиболее уязвимых по инфицированию и заболеванию категорий граждан. Общее число привитых против МИ в РФ в 2021 г. по сравнению с 2019 г. увеличилось на 31% (435 343 и 299 856 чел. соответственно), при этом доля вакцинированных детей увеличилась на 54% (в 2019 г. – 121 648 чел., в 2021 г. – 267 227 чел.). Согласно представленным данным, выявлен рост числа привитых, но охват населения вакцинацией остается недопустимо малым (0,3%) и не позволяет управлять эпидемическим процессом МИ, что является чрезвычайно опасным в настоящее время из-за значительного изменения эпидемиологии МИ и возможности начала очередного периодического подъема заболеваемости после 30-летнего межэпидемического периода. Отсчет продолжающегося в настоящее время межэпидемического периода ведется с 1991 г., когда показатель заболеваемости ГФМИ в РФ значительно снизился и достиг устойчиво низкого показателя заболеваемости (от 2,0 до 3,0 на 100 тыс. населения) вплоть до 2005 г. [20]. И только в последующие годы и до настоящего времени показатель заболеваемости не превышает уровня 2,0 на 100 тыс. населения, что, в соответствии с позицией ВОЗ, относится к низкому уровню эндемичности. Другие критерии, которые приводит ВОЗ, – это высокий уровень эндемичности (> 10 случаев на 100 тыс. населения) и промежуточный уровень эндемичности (2–10 случаев на 100 тыс. населения) [21].
Таким образом, в РФ с 2005 по 2016 г. показатели заболеваемости, согласно классификации ВОЗ, относились к категории низкой эндемичности и характеризовались устойчивой тенденцией к снижению, достигнув в 2016 г. 0,45 на 100 тыс. населения. Однако без видимых предвестников, неожиданно впервые за длительный период в 2017–2019 гг. показатель заболеваемости повысился с 0,48 на 100 тыс. населения в 2017 г. до 0,66 в 2019 г., но далее из-за жестких карантинных мер в отношении новой коронавирусной инфекции заболеваемость снизилась, составив в 2021 г. 0,22 на 100 тыс. населения (рис. 1).
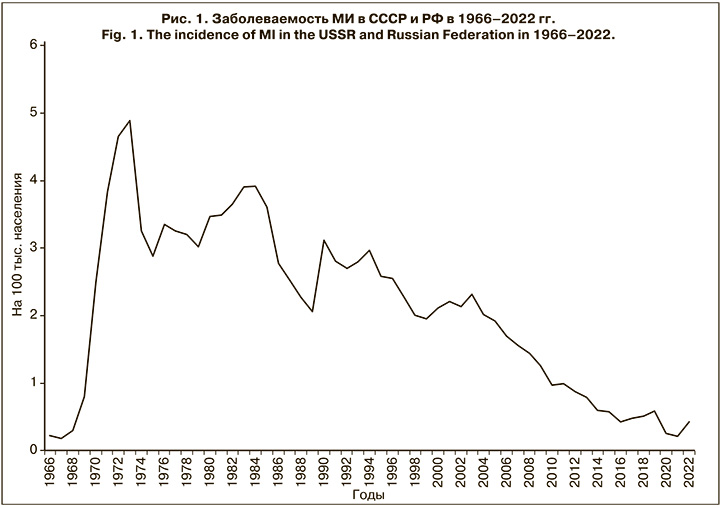
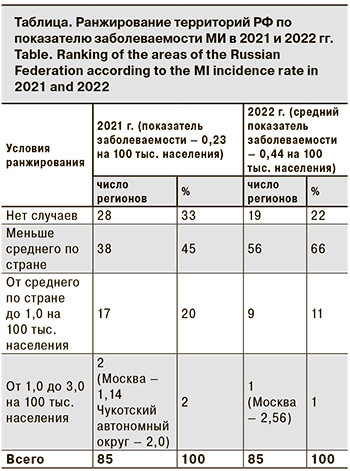
Следует отметить, что повышение заболеваемости в трехлетний доковидный период (2017–2019) сопровождалось необычными изменениями в эпидемиологии МИ. Так, возросла заболеваемость среди подростков и молодых взрослых (в 2,4 и 2,6 раза соответственно); увеличилась доля менингококков серогруппы А (в 5,5 раза); впервые за 23-летний период наблюдения в 2019 г. возник крупный очаг МИ с групповой заболеваемостью в г. Новосибирске. Выявленные изменения ряда эпидемиологических показателей указывали на признаки развивающегося осложнения эпидемической ситуации в отношении МИ, и в этой связи несомненный интерес представляет дальнейшее развитие событий и эпидемиологические проявления МИ в постковидный период. По данным Федерального статистического наблюдения «Сведения об инфекционных и паразитарных заболеваниях за 2022 год» (форма 2), заболеваемость МИ в РФ в 2022 г. возросла в 2,1 раза по сравнению с 2021 г., показатель составил 0,44 на 100 тыс. населения. Заболеваемость с разной степенью активности повысилась во всех федеральных округах РФ, кроме Дальневосточного, при этом в Южном федеральном округе она повысилась в 2,5 раза, в Центральном и Сибирском федеральном округах – в 2,3 раза, в Приволжском федеральном округе – в 1,8 раза. В других федеральных округах (Северо-Западном, Северо-Кавказском, Уральском) рост измерялся несколькими случаями. Ранжирование показателей заболеваемости по субъектам РФ показало, что на 19 (22,3%) территориях заболеваемость не регистрировалась, на 56 (66%) она была ниже среднероссийской (от 0,09 до 0,44 на 100 тыс. населения), а на 9 (10,6%) – выше среднероссийской (от 0,44 до 0,77 на 100 тыс. населения). При этом самый высокий показатель заболеваемости зарегистрирован в Москве – 2,56 на 100 тыс. населения, он превысил среднероссийский показатель почти в 6 раз (см. таблицу).
Интересно было изучить вклад возрастных групп на этапе повышения заболеваемости по показателям за 2022 г. На основании данных Федерального статистического наблюдения «Сведения об инфекционных и паразитарных заболеваниях за 2022 год» (форма 1) выявлено, что повышение заболеваемости по числу случаев произошло как в когорте детей до 17 лет (включительно), так и в когорте лиц старше 18 лет. Однако в 2022 г. по сравнению с 2021 г. число случаев в когорте детей до 17 лет возросло в 1,4 раза (с 183 до 259), а среди лиц от 18 лет и старше число случаев возросло в 2,9 раза (с 128 до 368). Аналогичные расчеты по региону Москвы показали, что повышение заболеваемости по числу случаев произошло как в когорте детей до 17 лет (включительно), так и в когорте лиц старше 18 лет. Однако в 2022 г. по сравнению с 2021 г. число случаев в когорте детей до 17 лет возросло в 1,6 раза (с 42 до 66), а среди лиц от 18 лет и старше – в 2,5 раза (с 101 до 254). Следовательно, заболеваемость в постковидный период повысилась в 2,1 раза, причем во всех федеральных округах (за исключением Дальневосточного федерального округа), территорией наибольшего риска МИ является Москва (показатель заболеваемости – 2,56 на 100 тыс. населения), преимущественно среди лиц от 18 лет и старше (в 2,9 раза в целом по стране и в 2, раза по Москве). Таким образом, есть основания предполагать, что эпидемическая ситуация по МИ будет осложняться и потребуются особая настороженность и внимание за эпидемиологическими проявлениями МИ на каждом территориальном образовании для экстренного проведения профилактических и противоэпидемических мероприятий в соответствии с СанПиН 3.3686-21 «Санитарно-эпидемиологические требования по профилактике инфекционных болезней» (раздел XXXIX «Профилактика менингококковой инфекции»). Главной профилактической мерой является упреждающая вакцинопрофилактика. В настоящее время в РФ зарегистрирован и разрешен для применения ряд вакцин, среди них А-менингококковая полисахаридная моновакцина (АО НПО «Микроген», Россия), А+С-менингококковая полисахаридная дивакцина (АО НПО «Микроген», Россия), АСWY – менингококковая конъюгированная четырехвалентная вакцина Menactra («Санофи Пастер», Франция) и В-менингококковая рекомбинантная белковая вакцина Bexsero (АО «ГлаксоСмитКляйн Трейдинг», Россия).
Заключение
В заключение на наглядной иллюстрации (рис. 2) уместно продемонстрировать чередование периодов подъема (20 лет) и спада (30 лет) заболеваемости МИ в историческом аспекте.

График впервые был продемонстрирован нами 15 ноября 2018 г. на 4-й Российской научно-практической конференции «Актуальные проблемы менингококковой инфекции и гнойных бактериальных менингитов» как предостережение по возможному неблагоприятному развитию эпидемиологических проявлений МИ. В настоящее время можно констатировать, что 30-летний межэпидемический период заканчивается и выявлены признаки начала очередного периодического подъема заболеваемости, прежде всего в этот процесс на данном этапе в наибольшей степени вовлечена Москва. Но это только начало. Чрезвычайно важными в этой связи являются бдительность и необходимость комплексной оценки текущей ситуации для осуществления мер упреждающей иммунопрофилактики в рамках региональных календарей профилактических прививок и своевременного начала расширенных профилактических и противоэпидемических мероприятий по эпидемическим показаниям в случае экстренного осложнения эпидемической ситуации.


