По данным ВОЗ, в мире отмечается четкая тенденция к росту числа больных хроническим гепатитом С (ХГС). Считается, что около 3% населения земного шара инфицированы вирусом гепатита С (ВГС), что составляет не менее 180 млн человек [1]. В Российской Федерации число лиц с хронической HCV-инфекцией составляет не менее 5 млн человек [2].
Сохранение высоких показателей заболеваемости ХГС в течение последних десятилетий способствовало увеличению частоты развития цирроза печени (ЦП). Риск развития ЦП при ХГС составляет от 15 до 56% на протяжении 20–30 лет течения инфекционного процесса. У пациентов с ЦП резко возрастает риск его декомпенсации и развития гепатоцеллюлярной карциномы (ГЦК) [3, 4]. Ежегодно от осложнений терминальной стадии ЦП в исходе ХГС умирают около 0,5 млн человек, ГЦК развивается у 1–5% больных ХГС [5, 6].
Процент пациентов с ЦП, нуждающихся в терапевтической и хирургической помощи, очень высок [7]. Известно, что 40% всех ортотопических трансплантаций печени проводится в связи с ЦП в исходе ХГС. Пациенты с сохраняющейся вирусной нагрузкой имеют высокий риск инфицирования донорской печени с возможным быстрым развитием ЦП или ГЦК в пересаженной печени, а также отторжения трансплантата [8].
 В настоящее время не вызывает сомнения тот факт, что эффективная противовирусная терапия (ПВТ) снижает риск прогрессирования заболевания печени [9, 10]. Тем не менее в связи с высоким риском серьезных осложнений лечение пациентов с декомпенсированным ЦП остается сложной задачей. Сложность заключается в том, что такие пациенты нуждаются в комплексной терапии, включающей, помимо ПВТ, коррекцию отечно-асцитического синдрома, печеночной энцефалопатии, профилактику кровотечений из варикозно-расширенных вен пищевода (ВРВП) и желудка, инфекционных осложнений и печеночно-клеточной недостаточности [11, 12].
В настоящее время не вызывает сомнения тот факт, что эффективная противовирусная терапия (ПВТ) снижает риск прогрессирования заболевания печени [9, 10]. Тем не менее в связи с высоким риском серьезных осложнений лечение пациентов с декомпенсированным ЦП остается сложной задачей. Сложность заключается в том, что такие пациенты нуждаются в комплексной терапии, включающей, помимо ПВТ, коррекцию отечно-асцитического синдрома, печеночной энцефалопатии, профилактику кровотечений из варикозно-расширенных вен пищевода (ВРВП) и желудка, инфекционных осложнений и печеночно-клеточной недостаточности [11, 12].
Современная ПВТ ХГС состоит из следующих слагаемых: максимальная эффективность (у разных групп пациентов, независимо от их исходных характеристик, в первую очередь при наличии ЦП), безопасность и переносимость (отсутствие или низкая частота нежелательных явлений – НЯ), приверженность к лечению, максимально короткий курс лечения, отсутствие или минимальные межлекарственные взаимодействия. Появление препаратов прямого противовирусного действия (ПППД) коренным образом изменило подход к ведению больных с тяжелым поражением печени (пациенты со значительным фиброзом и ЦП), включая больных ЦП классов В и С по Чайлду–Пью; пациентов с рецидивом HCV-инфекции после трансплантации печени; пациентов с клинически значимыми внепеченочными проявлениями (васкулит, криоглобулинемия, иммунокомплексная нефропатия, неходжкинская В-лимфома); больных на гемодиализе.
К настоящему времени накоплен большой опыт ведения и лечения пациентов с гепатитом С, который положен в основу клинических рекомендаций Европейской ассоциации по изучению болезней печени (EASL) по лечению пациентов с гепатитом С (2016), Российских рекомендаций по диагностике и лечению взрослых больных гепатитом С (2014), где приоритет в инициации ПВТ ПППД должен быть отдан пациентам с продвинутым фиброзом (F3) и ЦП. Крайне важно при выборе ПВТ учитывать наличие сопутствующих заболеваний и уже принимаемых лекарственных препаратов, с которыми у некоторых противовирусных препаратов могут возникнуть нежелательные лекарственные взаимодействия. Современные схемы ПВТ характеризуются высокой эффективностью (90–99 %) и безопасностью.
 Таким образом, ПВТ компенсированного и декомпенсированного ЦП в исходе ХГС замедляет процессы декомпенсации цирроза, снижает смертность от его осложнений, предотвращает развитие ГЦК, способствует профилактике реактивации HCV-инфекции после трансплантации печени, а также улучшает качество жизни больных.
Таким образом, ПВТ компенсированного и декомпенсированного ЦП в исходе ХГС замедляет процессы декомпенсации цирроза, снижает смертность от его осложнений, предотвращает развитие ГЦК, способствует профилактике реактивации HCV-инфекции после трансплантации печени, а также улучшает качество жизни больных.
Цель работы – оценка эффективности и безопасности ПВТ препаратами прямого противовирусного действия – паритапревиром/ритонавиром/омбитасвиром и дасабувиром – у пациентов с ЦП в исходе ХГС.
Материалы и методы
Под наблюдением находились 12 пациентов с ЦП, получавших ПВТ в период 2015–2016 гг. на базе Республиканской клинической инфекционной больницы им. проф. А.Ф. Агафонова (Казань).
Этиологическую верификацию проводили стандартными методами: ИФА с определением антител классов IgM и IgG cor, NS3, NS4, NS5 ВГС и ПЦР с подтверждением РНК ВГС и генотипированием. Диагноз ЦП устанавливали на основании клинических и лабораторных данных, результатов инструментальных методов обследования: УЗИ органов брюшной полости, ФЭГДС и эластометрии печени на аппарате «Фиброскан»® (FibroScan® компании «EchoSens», Франция) Оценку степени тяжести ЦП осуществляли по шкале Чайлда–Тюркотта–Пью (Child–Turcotte–Pugh).
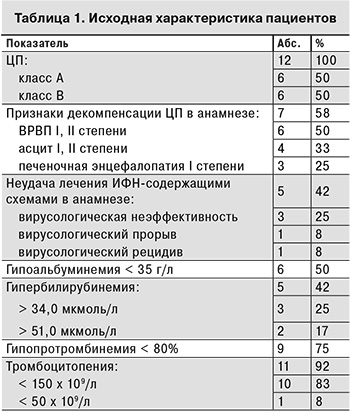
Исследуемую группу составили 5 (42%) мужчин и 7 (58%) женщин в возрасте от 38 до 74 лет (средний возраст – 51,8 ± 3,2 года), все были инфицированы ВГС генотипа 1b. 9 (75%) пациентов имели низкую вирусную нагрузку (< 800 000 МЕ/мл).
По 6 пациентов имели ЦП классов А и В (табл. 1). В практике не редкость случаи поздней диагностики ХГС, уже на стадии ЦП. Так и в данной группе у 3 пациентов вирусная этиология заболевания была установлена на стадии ЦП, причем у 1 пациента – при клинической манифестации признаков декомпенсации (асцита).
До начала терапии 11 из 12 больных предъявляли жалобы астеновегетативного характера на слабость, повышенную утомляемость. Кроме того, 7 пациентов отмечали боли в правом подреберье, 5 – желтуху, в том числе 2 – кожный зуд. 7 пациентов имели признаки декомпенсации ЦП, такие как ВРВП, асцит, печеночная энцефалопатия. 9 пациентов имели сопутствующие заболевания: сахарный диабет 2-го типа (2), ревматизм (2), гипертоническую болезнь (2), аутоиммунный тиреоидит (1), кисту почки (1), В-клеточную лимфому с криоглобулинемическим васкулитом (1).
Согласно результатам лабораторных анализов, у обследованных пациентов минимальные показатели уровня альбумина составили 29,8 г/л, тромбоцитов – 42 х 109/л, протромбинового индекса – 61%; максимальный уровень билирубина – 77,96 мкмоль/л.
Все пациенты получали паритапревир/ритонавир/омбитасвир (75 мг/50 мг/12,5 мг) по 2 таблетки 1 раз в сутки и дасабувир (250 мг) по 1 таблетке 2 раза в сутки на протяжении 12 недель.
Оценку вирусологической эффективности и безопасности ПВТ проводили в конце 4-й, 8-й и 12-й недели терапии и через 12 и 24 недели после ее завершения. Достижение неопределяемого уровня РНК ВГС на 4-й, 8-й, 12-й неделях терапии расценивалось как вирусологический ответ (ВО), на 12-й и 24-й неделях после завершения лечения – как устойчивый вирусологический ответ (УВО12 и УВО24 соответственно).
Результаты
В настоящее время все 12 пациентов завершили 12-недельный курс терапии, 8 из них прошли период наблюдения 12 недель после лечения, в том числе 6 – период наблюдения 24 недели после лечения.
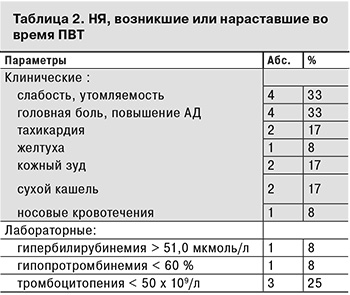
Во время ПВТ НЯ отмечены у 8 (66,7%) пациентов (табл. 2).
Чаще всего пациенты предъявляли жалобы астеновегетативного характера на слабость, утомляемость, а также на головную боль, повышение АД. У одного пациента на 8-й неделе ПВТ появилась желтуха с гипербилирубинемией до 53,8 мкмоль/л и протромбиновый индекс снизился до 52%. Еще у 3 больных была отмечена тромбоцитопения < 50 х 109/л.
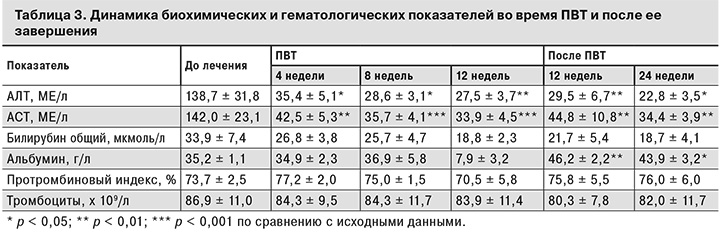
Изменения лабораторных показателей во время терапии и после ее завершения имели разноплановый характер (табл. 3). Наблюдалась положительная динамика снижения к концу 4-й недели и нормализация к концу 8-й недели терапии уровней трансаминаз (АЛТ, АСТ), повышение и нормализация после лечения уровня альбумина – показателя состояния белковосинтетической функции печени. Как во время терапии, так и после ее завершения мы не отметили достоверных изменений других биохимических и гематологических показателей по сравнению с исходными данными. Однако у части пациентов наблюдали разрешение желтухи и нормализацию уровня билирубина (у 3 больных из 5), восстановление уровня тромбоцитов (у 1 больного из 11).
Нами не было отмечено ни одного случая серьезного НЯ. Возникшие во время ПВТ НЯ разрешились в период наблюдения после завершения лечения.
ПВТ комбинацией препаратов паритапревир/ритонавир/омбитасвир и дасабувир показала высокую эффективность. ВО получен на 4-й неделе лечения у 8 (66,7%) больных, на 8-й и 12-й неделях – у всех пациентов. Кроме того, УВО12 достигнут у 8 (100%) пациентов, в том числе у 6 (100%) – УВО24.
Обсуждение
Несомненно, в настоящее время достигнут большой успех в лечении ХГС. Это связано с появлением новых ПППД. Их использование по сравнению со схемами, включающими ИФН, позволило повысить вирусологическую эффективность и улучшить профиль безопасности терапии, в том числе у «сложных» пациентов, в первую очередь с ЦП. Все это убедительно продемонстрировано в многочисленных клинических исследованиях.
Особого внимания заслуживает исследование TURQUOISE II [13] как самое многочисленное, посвященное изучению эффективности и безопасности терапии, включающей паритапревир/ритонавир/омбитасвир и дасабувир в комбинации с рибавирином, в течении 12 или 24 недель у 380 пациентов с компенсированным ЦП и генотипом 1 ВГС. В целом УВО12 был получен у 92% больных после 12-недельного курса лечения и у 96% – после 24-недельного. Наилучшие результаты были продемонстрированы у пациентов с ВГС генотипа 1b: УВО12 достигнут у всех ранее не леченных пациентов и у 98,7% пациентов, ранее не ответивших на ПВТ схемами, содержащими ИФН. Наиболее частыми НЯ были утомляемость, головная боль и тошнота. Серьезные НЯ наблюдались у 5–6% больных, развитие НЯ стало причиной отмены терапии у 1,9–2,3 % больных.
В исследовании TURQUOISE III [14], куда были включены пациенты с ЦП класса А и ВГС генотипа 1b, также была показана высокая эффективность 12-недельного курса ПВТ паритапревиром/ритонавиром/омбитасвиром и дасабувиром: у всех 60 пациентов был получен УВО12.
Полученные результаты собственного наблюдения можно расценивать как сопоставимые с данными международных клинических исследований. Причем половина группы (6 пациентов) имела признаки декомпенсированного ЦП класса В. Следует пояснить, что часть больных получала лечение в 2015 г. до появления ограничений по применению комбинации препаратов паритапревир/ритонавир/омбитасвир и дасабувир у пациентов с декомпенсированным ЦП класса В, другая – по жизненным показаниям. Лечение больных с ЦП – непростая задача и определяется не только ПВТ, направленной на устранение этиологического фактора заболевания. Не менее важным является проведение других лечебных мероприятий, как консервативных (патогенетическая терапия осложнений ЦП), так и оперативных (при наличии показаний – трансплантация печени). Возможно, после успешной ПВТ у части пациентов будет отсрочен вопрос трансплантации печени, у части – минимизированы риски прогрессирования заболевания, развития НЯ, связанные с иммуносупрессивной терапией после трансплантации.
Заключение
ПВТ паритапревиром/омбитасвиром/ритонавиром и дасабувиром показала высокую эффективность у пациентов с ЦП классов А и В: у всех пациентов получен ВО на 8-й неделе и на момент завершения терапии, у 8 (100%) – УВО12 и у 6 (100%) – УВО24. В целом была отмечена удовлетворительная переносимость, за время терапии не было зарегистрировано серьезных НЯ, а наблюдаемые НЯ не потребовали досрочного завершения лечения.


